Home > API Reference Standards > [124959-28-0]2-chloro-4-(furan-2-yl)pyrimidine
Product Description
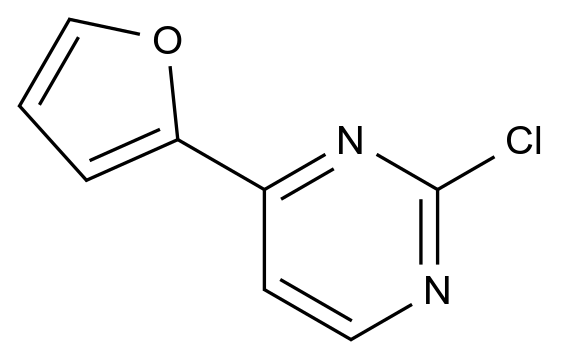
English name: 2-chloro-4-furan-2-yl-pyrimidine; 2-Chloro-4-(fur-2-yl)pyrimidine; 2-chloro-4-(2-furanyl)pyrimidine; 2-(2-chloropyrimidin-4-yl)furan; 2-chloro-4-(2-furyl)pyrimidine; Pyrimidine,2-chloro-4-(2-furanyl)
Chinese name: 2-氯-4-(呋喃-2-基)嘧啶
CAS Number: 124959-28-0
Molecular Formula: C8H5ClN2O
Molecular Weight: 180.591
boiling point:342.3oC at 760mmHg
melting point: 81.5oC
flash point: 160.8oC
psa: 38.92
logp: 2.39
In order to ensure the safety of 2-chloro-4-(furan-2-yl)pyrimidine transport process, we use high standard production product packaging, tested can achieve 371 metres drop protection effect.
※High-strength PP material product liner tube, with high impact resistance, strong mechanical properties, resistant to a variety of organic solvents and acid and alkali corrosion;
※The liner tube is made of anti-theft cap, with a ring-shaped anti-leakage ring on the top inner wall and an anti-theft ring attached to the bottom end to ensure that the product is opened for the first time;The product label is made of PP synthetic paper, which is tear-proof, break-proof and moisture-proof;
※The interior of the product is filled with sponge, shock-absorbing and anti-friction.

Product 2-chloro-4-(furan-2-yl)pyrimidine of CATO Reference Standards is an analytical standard developed under the ISO 17034 standard substance producer competence certification system.
As a necessary quality standard in the process of drug quality analysis, the accuracy of its measurement value is related to drug safety. Currently, most of the drug standard products are only accompanied by simple product quality instructions, in order to ensure the accuracy of drug research, some testing laboratories need to consume a part of the product, the use of pharmacopoeia specified methods for repeated testing and verification of purity, consuming manpower and material resources.
Meanwhile, drug impurity research is an important part of drug evaluation. The Code of Practice and Technical Requirements for Drug Registration and Inspection (for Trial Implementation) (2020 Edition), issued by the Chinese Academy of Inspection and Quarantine (CAIQ), clearly stipulates that "the applicant should provide the appropriate standard substances and research information when applying for registration and inspection, and the quantity of the standard substances provided should be able to satisfy the inspection demand". Insufficient impurity studies have become a common factor leading to the failure of drug reviews.
CATO Technology is the first enterprise in China to pass the ISO17034 international standard substance production qualification operation, and is also the only standard sample development enterprise in the world that has passed the domestic and international dual ISO17034 system certification, and has the qualification to develop the whole category of organic analytical standard samples in the form of pure products and standard liquid products. (Certification enquiry website: ANAB: http://search.anab.org/; CNAS: https://www.cnas.org.cn/rkcx/2013/03/728834.shtml.)
Through the ISO17034 system, CATO has established an excellent quality control system for standard samples, covering production planning, candidate preparation, homogeneity study, stability study, determination of eigenvalues, licensing, inventory, product sales, product use tracking and technical support, etc. CATO standard samples have fully met the strict requirements of homogeneity, stability and traceability required by the system, and have successfully assisted many pharmaceutical enterprises to develop standard samples. CATO standard products fully meet the strict requirements of homogeneity, stability and traceability, and have successfully helped many pharmaceutical customers to pass the drug marketing approval.
CATO standard products will provide a full set of product quality specifications, including charts, structural conformity report, etc., the specific testing methods include NMR, mass spectrometry, liquid phase, gas phase, infrared, ultraviolet, moisture, incandescent residue, etc., eliminating the need for customers to reconfirm the receipt of the goods, and at the same time, structural conformity report can be used as a supporting material for evaluation of the quality of the study of the drug.

Technical Information
Tel: 13342851930
Fax: +86-20-81717260
E-mail: tianwen.zhan@cato-chem.com
Add: Add: 3rd Floor, Building B, No.179, Guangpu Rd East, Huangpu Dist, Guangzhou , Guangdong, China
Consulting Questions